Abstract
Introduction: Autologous Hematopoietic Cell Transplantation (AHCT) remains the standard of care for multiple myeloma (MM) patients who achieve at least a partial response to prior chemotherapy. Survival post AHCT is associated with disease risk and patient comorbidities, including lung diseases indicated by pulmonary function. The corrected diffusing capacity for carbon monoxide (DLCOc) is usually assessed in transplant candidates in order to determine AHCT eligibility, although precise DLCOc cut-offs are yet to be standardized. Recent studies suggested that a DLCOc ≤60% possesses a prognostic value in predicting relapse and mortality post AHCT. Hence, we aimed to validate the role of DLCOc ≤60% in predicting post-AHCT outcomes at our institution.
Methods: We did a retrospective review of MM patients who underwent AHCT at Cleveland Clinic between January 1st, 2011 and January 15th 2021. The patients were categorized based on their corrected DLCO into two groups: DLCOc ≤60% and DLCOc >60% of the predicted value. Baseline characteristics, including patient, disease, and transplant factors were compared between the two groups. The collected data were analyzed using SPSS and GraphPad Prim by means of Chi square, ANOVA, and Kaplan Meier survival analysis.
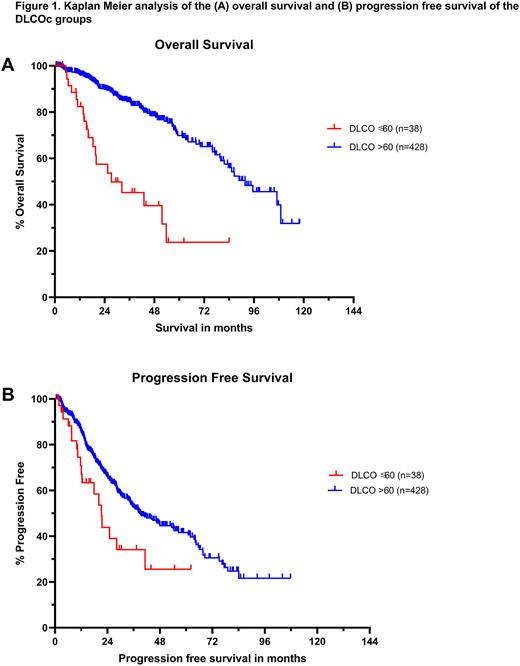
Results: A total of 466 patients with available DLCOc data were included in our study, of which 38 patients had a DLCOc ≤60% and 428 had a DLCOc >60% of the predicted value. Our cohort had a median follow up of 31.9 months post-AHCT. Patient and disease characteristics were similar between the 2 groups, including age (median 59.1 vs 60.3 years, P= 0.407), sex (Males 65.8 vs 58.2%, P=0.361), standard risk (81.5 vs 66.4%) and high risk (18.5 vs 33.6%) cytogenetic profiles (P=0.107), as well as history of smoking (23 vs 38.2%, P=0.1). In addition, both groups had a similar distribution across the International Staging System (ISS) stages (37.9, 37.9, 24.2% vs 38.3, 38.9, 22.8% for stages I, II and III, P= 0.987) and received similar preparative regimens (92.1, 7.9, 0% vs 96.3, 3, 0.7% for Melphalan 200 mg/m2, Melphalan 140 mg/m2 and Busulfan + Cyclophosphamide, P=0.25). Kaplan-Meier survival analysis showed that the DLCOc ≤60% group survived for a significantly shorter period with median overall survival (mOS) of 27.3 vs 91.7 months (P<0.0001) (Fig 1A). In addition, the DLCOc ≤60% group had earlier relapses with median progression free survival (mPFS) of 21.2 vs 38.7 months (P=0.0115) (Fig 1B). Subgroup analysis confirmed that mOS was significantly shorter in the DLCOc ≤60% group irrespective of relapse, with mOS of 25.4 vs 79.6 months (P<0.0001) in relapsed MM of DLCOc ≤60% (n=18) and DLCOc >60% (n=193) patients, respectively. Similarly, non-relapse survival was also shorter (medians unreached) in the non-relapsed DLCOc ≤60% group (n=20) compared to the non-relapsed DLCOc >60% group (n=235) (P<0.0001). When selecting patients of standard risk cytogenetics from both groups, the significant difference in mOS and mPFS was retained with mOS 51.7 vs unreached months (P<0.0001) and mPFS 21.2 vs 67.7 months (P=0.0051) for DLCOc ≤60% group (n=22) and DLCOc >60% (n=225), respectively. Likewise, matching for melphalan 200 mg/m2 regimen patients further maintained the remarkable gap in mOS 25.4 vs 91.7 months (P<0.0001) and mPFS 20 vs 40 months (P=0.0013) for DLCOc ≤60% group (n=35) and DLCOc >60% (n=413), respectively.
Conclusion: The pre-AHCT DLCOc level possesses a useful prognostic value that can predict disease relapse as well as both relapse- and non-relapse related mortality post AHCT. This was demonstrated when using a cut-off value of ≤60% vs >60%. Our findings were supported by the fact that both groups had similar clinical and cytogenetic characteristics, thus reducing the risk of confounding effects. Our findings were also validated when controlling for cytogenetic risks and melphalan dosage. Large, prospective trials are warranted to determine whether DLCOc ≤60% can serve AHCT eligibility criterion.
Disclosures
Jagadeesh:Trillium Pharmaceuticals: Research Funding; AstraZeneca: Research Funding; Debio pharma: Research Funding; Regeneron Pharmaceuticals, Inc.: Research Funding; Seagen: Research Funding; MEI Pharma: Research Funding; LOXO Pharmaceuticals: Research Funding; ATARA Biotherapeutics: Research Funding; Daiichi Sankyo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Affimed: Membership on an entity's Board of Directors or advisory committees. Winter:OncLive: Honoraria; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Seagen, Inc: Consultancy, Membership on an entity's Board of Directors or advisory committees. Sauter:BMS: Other: PI; Kite Pharma Inc.: Consultancy; Precision Biosciences: Other: PI; Karyopharm Therapeutics Inc.: Consultancy; Ono Pharmaceuticals: Consultancy; Gamida Cell: Consultancy; CSL Behring: Consultancy; Genzyme/Sanofi: Other: PI. Hill:BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support, Research Funding. Valent:Alexion, AstraZeneca Rare Disease: Research Funding. Hamilton:Equilium: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Syndax: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees; Kadmon: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.